Parkinson’s disease (PD) is a neurodegenerative disorder characterized by tremors of the fingers, hands, head, and neck, bradykinesia (slowed movements), and rigidity of muscles. Approximately 1.5 million Americans are living with PD, and as many as 95,000 new cases are reported in the country each year [1]. PD-related costs to Medicare, which covers 90% of Americans with PD, are approximately $10,000/patient/year with a total economic burden of over $50B/year [2–4]. PD treatments include pharmaceutical interventions such as levodopa and surgical procedures such as deep brain stimulation (DBS) and drug pumps.
Treatment must be customized for each patient, with optimal quality of life being the most important goal. For 10-15 years in the early stages of the disease after starting a pharmaceutical intervention, patients work with their healthcare provider to manage motor complications by adjusting medication to maintain stable symptom control. Wearing off, a common problem in PD patients who have used levodopa for several months or years, is the reemergence of symptoms a few hours after taking medication but before the next scheduled dose [5]. Some patients experience delayed ON, a prolonged time to experience improvement in symptoms after a treatment dose [6]. In addition, involuntary movements known as dyskinesias can occur when dopamine stimulation in the brain is at a maximum [6]. Managing fluctuations to minimize symptoms without causing dyskinesias continues to be a daunting task. The patient is in the middle of a complex system where drug types, dose levels, and times interact to create patterns of motor symptoms and side effects fluctuating throughout the day. Tools for patients to monitor, let alone act on, these temporal patterns are severely lacking.
Subjective clinical rating scales are used to track symptoms during routine clinical management and in clinical trials. Ratings scales, however, require the presence of a clinician, which does not lend itself to monitoring symptom fluctuation patterns throughout the day. Obtaining only a snapshot of symptoms during a single clinical office visit once or twice a year does not provide enough time resolution to determine how to optimize symptomatic benefit. In addition to these clinical evaluations, patients are often asked to keep a diary of their symptoms throughout the day. Paper diaries, however, can be burdensome, leading to poor compliance and inaccuracies [7]. Additionally, patients are often unable to recognize when they are not responding optimally and are unaware of what changes they can make themselves to improve treatment. These limitations can make decisions about medication adjustments particularly challenging and require costly trial-and-error to determine what works best. And while telehealth became more popular to reduce office visits during the COVID-19 pandemic, remote video assessment still only provides a snapshot and is often inadequate due to poor video quality [8].
Remote monitoring with wearables has shown great promise in providing objective evidence for clinical decision-making and helping PD patients better understand how their choices can impact their disease management. To that end, Great Lakes NeuroTechnologies developed the FDA-cleared and CE-marked KinesiaU™ motor assessment system, which uses a smartphone and consumer smartwatch (e.g., Apple Watch, Samsung Galaxy Watch) to continuously collect motion data from the watch’s accelerometers and gyroscopes for monitoring PD symptoms and medication side effects (Figure 1). The system analyzes motion data collected from the smartwatch throughout the day during daily activities using previously validated algorithms [9,10] to track tremor, slowness, and dyskinesia every two minutes, as well as therapies and activities in user-friendly reports. These can help patients and clinicians make better care decisions, identify therapies and activities to improve their symptoms, and lead to faster therapy optimization. Use of the KinesiaU system is billable as part of remote patient monitoring [14].
In a study completed with UCB Biopharma, patients who used an early version of KinesiaU to receive feedback on their symptom severities in real-time, and whose doctors used the data to adjust medication dosage, had significantly better clinical outcomes compared to patients receiving standard care [11]. In another study, KinesiaU collected data from patients before and after a change in therapy regimen [10]. Data recorded by the system detected changes in PD symptoms within a day and across days, identified medication side effects, recognized when medication wore off, and provided information to help clinicians optimize therapy regimens. Most recently, KinesiaU was used over an 8-month period by advanced PD patients recruited from the Cleveland Clinic and University of Cincinnati to determine if it could help identify patients who would be good candidates for advanced therapies such as DBS and drug pumps [12]. Artificial intelligence (AI) models used the data collected from KinesiaU to successfully identify DBS candidates and detect improvements resulting from DBS therapy.
The above examples demonstrate several clinical applications of remote monitoring in PD. Monitoring patients remotely reduces disparities as patients living in rural areas often have significantly worse quality of life than their urban counterparts [13]. With remote monitoring, patients can be monitored at home and only travel when a clinic visit is truly necessary. Remote monitoring also provides comfort to patients with the knowledge that they can continually be monitored by their healthcare providers after starting a new therapy. Additionally, remote monitoring can expand access to advanced therapies for patients who live far away from specialty clinics, live in underserved communities, or otherwise have limited access to a specialist and may not have advanced therapy. Objectively screening patients at home reduces the need for travel to a PD specialty center until after being identified as a suitable candidate for advanced therapy.
Remote monitoring can also help patients advocate for themselves. People with PD and their caregivers often struggle when trying to express their daily experiences with their doctors. This may also cause frustration for caregivers when the patient’s symptom experience in the clinic does not reflect what they see at home. Patients being able to show their doctors objective evidence of our they are doing home validates their experience. This empowers patients and their caregivers to take ownership of treatment and improve their quality of life.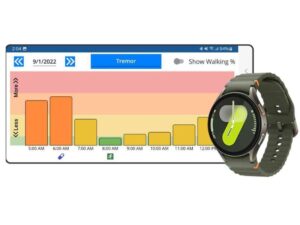
Figure 1. KinesiaU motor assessment system.
Works Cited
- Willis AW, Roberts E, Beck JC, Fiske B, Ross W, Savica R, Van Den Eeden SK, Tanner CM, Marras C, Alcalay R, Schwarzschild M, Racette B, Chen H, Church T, Wilson B, Doria JM. Incidence of Parkinson disease in North America. Npj Park Dis 2022 81. 2022 Dec 15;8(1):1–7.
- Dahodwala N, Li P, Jahnke J, Ladage VP, Pettit AR, Kandukuri PL, Bao Y, Zamudio J, Jalundhwala YJ, Doshi JA. Burden of Parkinson’s Disease by Severity: Health Care Costs in the U.S. Medicare Population. Mov Disord. 2021 Jan;36(1):133–42.
- Pearson C, Hartzman A, Munevar D, Feeney M, Dolhun R, Todaro V, Rosenfeld S, Willis A, Beck JC. Care access and utilization among medicare beneficiaries living with Parkinson’s disease. Npj Park Dis. 2023 Jul 10;9(1):1–7.
- Yang W, Hamilton JL, Kopil C, Beck JC, Tanner CM, Albin RL, Ray Dorsey E, Dahodwala N, Cintina I, Hogan P, Thompson T. Current and projected future economic burden of Parkinson’s disease in the U.S. Npj Park Dis. 2020 Jul 9;6(1):1–9.
- Hauser RA. Long-term care of Parkinson’s disease. Strategies for managing “wearing off” symptom re-emergence and dyskinesias. Geriatrics. 2006 Sep;61(9):14–20.
- Mestre T, Ferreira JJ. Pharmacotherapy in Parkinson’s disease: case studies. Ther Adv Neurol Disord. 2010 Mar;3(2):117–26.
- Papapetropoulos SS. Patient Diaries As a Clinical Endpoint in Parkinson’s Disease Clinical Trials. CNS Neurosci Ther. 2012;18(5):380–7.
- Park KW, Wu HJ, Yu T, Mahal R, Mirian MS, McKeown MJ. Potential Pitfalls of Remote and Automated Video Assessments of Movements Disorders. Mov Disord Off J Mov Disord Soc. 2023 Mar 27;38(3):504–6.
- Pulliam CL, Heldman DA, Brokaw EB, Mera TO, Mari ZK, Burack MA. Continuous assessment of levodopa response in Parkinson’s disease using wearable motion sensors. IEEE Trans Biomed Eng. 2018;65(1).
- Hadley AJ, Riley DE, Heldman DA. Real-World Evidence for a Smartwatch-Based Parkinson’s Motor Assessment App for Patients Undergoing Therapy Changes. Digit Biomark. 2021;5(3):206–15.
- Isaacson SH, Boroojerdi B, Waln O, McGraw M, Kreitzman DL, Klos K, Revilla FJ, Heldman D, Phillips M, Terricabras D, Markowitz M, Woltering F, Carson S, Truong D. Effect of using a wearable device on clinical decision-making and motor symptoms in patients with Parkinson’s disease starting transdermal rotigotine patch: A pilot study. Parkinsonism Relat Disord. 2019 Jul;64:132–7.
- Fadil R, Walter B, Kuhlman G, Zaidi S, Heldman DA. Smartwatch-Based Screening to Identify Candidates for Deep Brain Stimulation in Parkinson’s Disease. Mov Disord. 2024;39(suppl 1).
- Klepac N, Pikija S, Kraljić T, Relja M, Trkulja V, Juren S, Pavliček I, Babić T. Association of rural life setting and poorer quality of life in Parkinson’s disease patients: A cross-sectional study in Croatia. Eur J Neurol. 2007 Feb;14(2):194–8.
- Great Lakes NeuroTechnologies and ZS plan reimbursement for monitoring Parkinson’s disease [Internet]. ZS Digital Health Case Studies. [cited 2025 Apr 1]. Available from: https://www.zs.com/about/case-studies/great-lakes-kinesiau-parkinsons-disease-reimbursement-strategy




